Lignin is a second component of lignocellulose. This polymer is a phenolic polymer with high degree of cross-linkage. The three main monomers of lignin are p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol (Scheme 2). As such, lignin is a precious source of non-fossil aromatic, the only direct alternative to petrochemical industry. However for a long time it was treated as a by-product of the pulping industry and burnt.
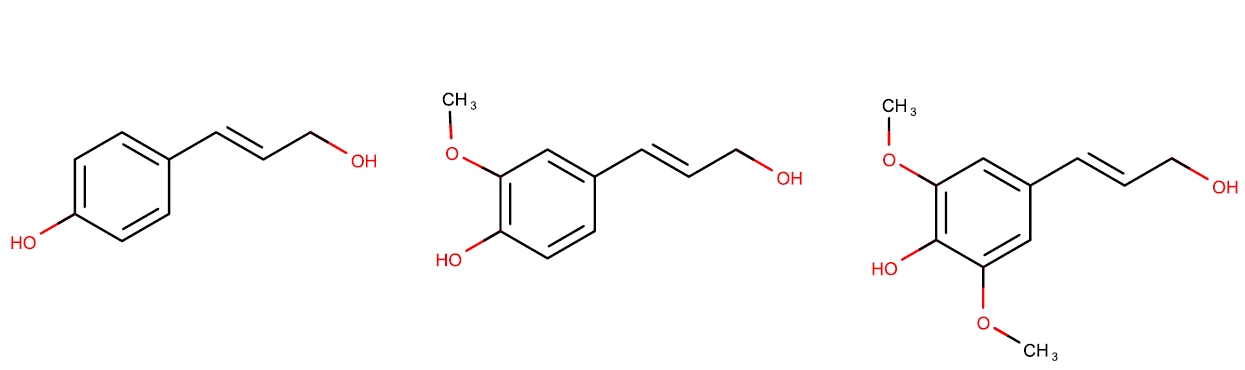
Scheme 2. From left to the right: p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol.
As mentioned above, lignin is solubilized in the same solvents, as cellulose. But selective solubility of lignin can be achieved by careful selection of the solvent system. For example, choline based ILs with carboxylate anions mixed with water dissolved lignin efficiently in mild conditions and solubility increased with increase of alkyl chain on the anion. Furthermore, precipitation of reconstructed lignin could be achieved by addition of more water.[i] On the other hand, acidic or basic ILs could be used as catalysts or catalysts and solvents for depolymerisation of lignin. Water is required in those systems as a co-solvent: to set pH of the Brønsted acidic IL solutions for more controlled reaction outcome and to improve interaction between lignin and IL in case of basic ILs.[ii] It must be stated though that the recovery of the ILs for the reuse from lignin solution is the factor for further improvement. Furthermore, the ideal case would suggest tandem delignification-depolymerization of lignin from biomass to better harness the full potential of lignin.
