Metals play a vital role in modern technology-based society. While some of them are comparatively easy to mine, some other metals are very scarce and dispersed. A lot of metals nowadays are simply disposed of as a part of waste. However, ever-growing population of our planet will demand more efficient use of resources and a switch from cradle-to-grave material flow to circular economy would have to be performed. In this setup, recovery of metals from complex matrices, like electronic waste, nuclear waste, industrial water streams and soil will increasingly gain importance. In addition, efficient metal extraction methods would facilitate mining when metal-rich ores are depleted.
Ionic liquids have ability to dissolve a wide spectrum of materials and therefore offer a good alternative to the current liquid-liquid extraction based metal recovery methods. ILs possess the following properties, that make them good candidates for the metal extraction:
- Low volatility: eliminates material loss due to evaporation and escape to the environment and avoids emission of VOCs;
- Non-flammability: minimizes fire and explosion hazards;
- Good stability (both thermal and electrochemical): lowers losses due to decomposition and allows for various treatment of metal solutions;
- Hydrophobicity: Various degrees make ILs compatible for extraction from aqueous phase;
- High solubilizing power: allows high efficiency of extraction;
- Highly tuneable properties: ILs could be used neat or in combination with chelating agents. In addition it is possible to introduce functional groups into both cation and anion (“Task-Specific-ILs”, TSILs) offering an opportunity to achieve high selectivity for the extraction of a given material;
Rare earth elements (REE), precious metal extractions have been studied with ILs. Typically, hydrophobic ILs are used, but extraction becomes more difficult with the increase of the hydrophobicity of the IL cation, when non-functionalized ILs are applied. In addition, extraction with non-functionalized ILs often proceeds via ion exchange mechanism, when cation of the metal to be extracted forms a complex with anions of IL and therefore, moves to IL phase, whereas IL-cations take metal´s place in aqueous phase. This results in continuous loss of IL and contamination of the aqueous phase (Scheme 1, a).
On the other hand, it is possible to use ILs in a mixture with chelating agents bearing neutral donor atoms (e.g. phosphorous, nitrogen, sulphur), which facilitate formation of hydrophobic metal-containing species and transport of the metals into the IL phase (Scheme 1, b). The stripping of metal from ILs afterwards can be easily performed by acidic treatment.[i]
Finally, task-specific ionic liquids (TSILs) could be used for the metal extraction.[ii] TSILs are functionalized ionic liquids, where cation or/and anion bear groups, that can carry out a specific task, in this case the coordination of metals. Therefore, TSIL combine the roles of extracting fluids and the ligand/chelating agents (Scheme 1, c). Furthermore, the properties of the functional group (basicity, geometry, affinity to a certain species) can be altered in a way that allows very high selectivity for the extraction of specific metals.
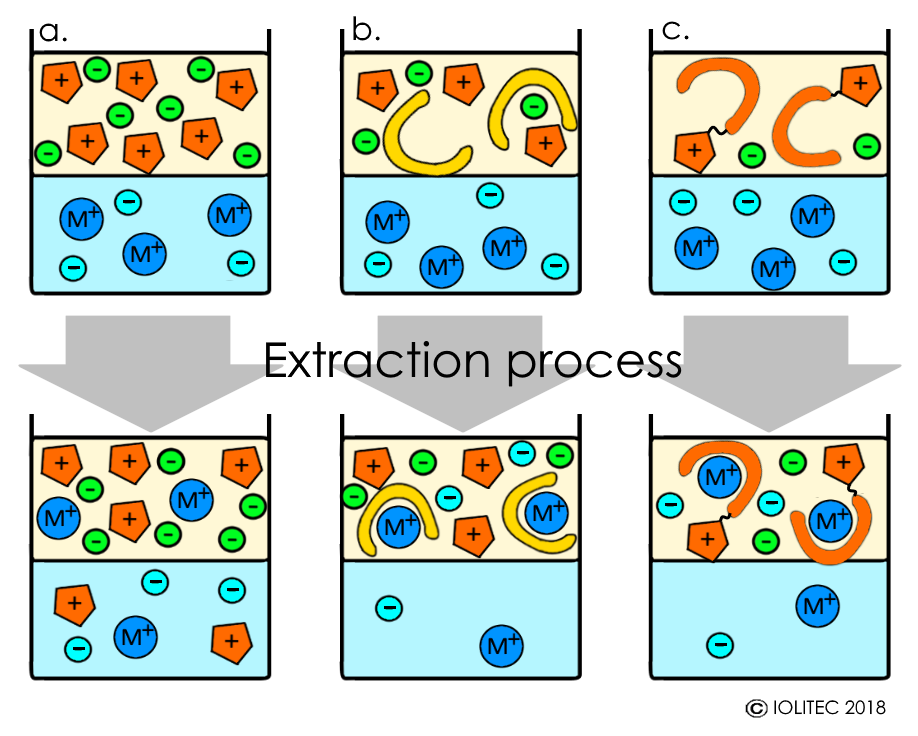
Scheme 1. Extraction of metals with ILs: a. using non-functionalized ILs; b. using a mixture of non-functionalized IL and a chelating agent; c. Using TSILs.
Do you have interest in using ILs for extracting metals? Please feel free to contact us for more information on the topic.
Text and illustration: Dr. Svetlana Cadu, 2018
[i] A. E. Visser, R. P. Swatloski, S. T. Griffin, D. H. Hartman, R. D. Rogers, Sep. Sci. Technol. 2001, 36, 785.
[ii] A. Quadi, B. Gadenne, P. Hesemann, J. J. E. Moreau, I. Billard, C. Gaillard, S. Mekki, G. Moutiers, Chem. Eur. J. 2006, 12, 3074.
