There are two different mode of absorption of CO2 by ILs.
- Physical absorption occurs, when CO2 fills the voids between the molecules of IL and is held there by Coulombic interaction. The advantage of this mode of capture is the ease of release of the stored CO2, since it is only weakly bound. The best ILs for physical absorption of CO2 have (partially) fluorinated anions[1] and cations with long alkyl substituents.[2] Among them, P666(14) BTA and P666(14) Cl, possess one of the highest abilities for physical absorption of CO2.[3]
- During chemical absorption, a reaction between CO2 and functional group of ionic liquid takes place and adduct of a certain stoichiometry is formed. The functional groups on those task specific ionic liquids (TSILs) include amino-, hydroxyl-, fluoro- and carbonyl groups. For example, reaction between amino functionality on cation of IL with CO2 leads to the formation of carbamate zwitterion, and 2 of such cations are necessary for reaction with one molecule of CO2 (1:2 stoichiometry).[4] On the other hand, when anion has an amino group (e.g. amino acid based ILs), carbamic acid is formed and reaction follows 1:1 stoichiometry (Scheme 1).[5] Furthermore, often after saturation of the chemical absorption capacity, physical absorption takes place resulting in overall CO2 absorption >1 molCO2/molIL.
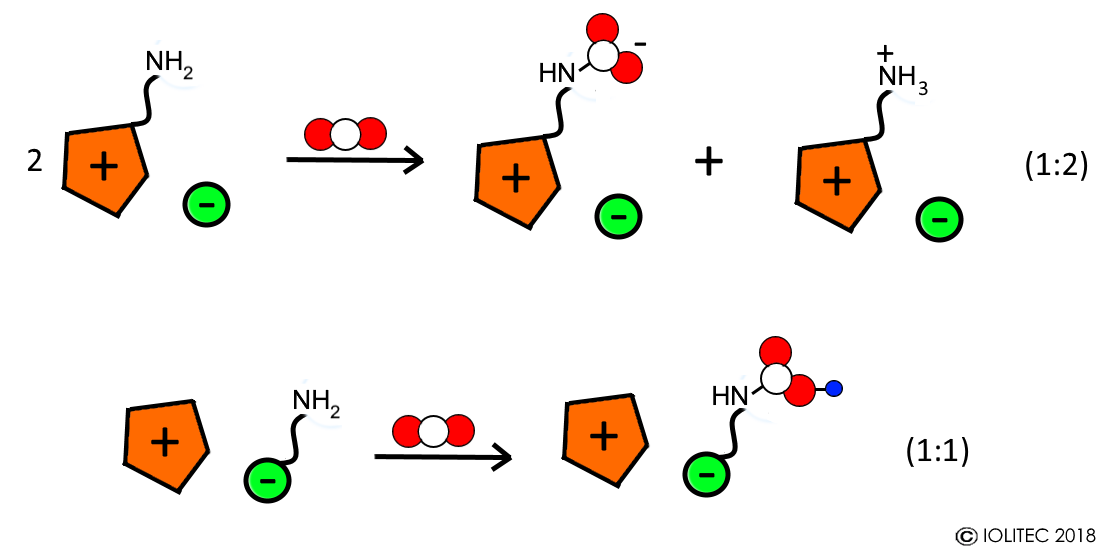
Scheme 1. Chemical absorption of CO2 with ILs functionalized with amino groups in cation and anion.
Most interestingly, while normally chemical absorption results in the viscosity increase of the IL, there are cases, e.g. P666(14) Im, for which viscosity drops upon CO2 dissolution.[6]
One intriguing effect while using IL-water mixture is when IL acts as catalyst for bicarbonate formation between CO2 and water. In case of BMIM Im over 10 molCO2/molIL absorption capacity can be achieved.[7]
IOLITEC has investigated a series of ILs capable of chemical absorption for CO2 in the joint project DIACAT (Scheme 2). The results there suggest that for given ILs, the optimal efficiency is obtained when used in the mixture with diethanolamine and water.
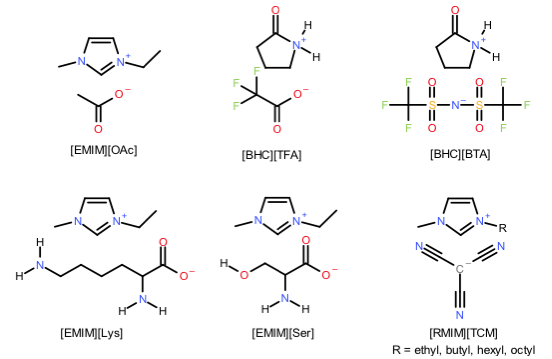
Scheme 2. ILs tested in the project.
If you want to discuss this topic further, please do not hesitate to contact us.
Text and illustration: Dr. Svetlana Cadu, 2018
1 S. N. V. K. Aki, B. R. Mellein, E. M. Saurer, J. F. Brennecke, J. Phys. Chem. B 2004, 108, 20355.
[2] J. L. Anthony, J. L. Anderson, E. J. Maginn, J. F. Brennecke, J. Phys. Chem. B 2005, 109, 6366.
[3] P. J. Carvalho, V. H. Alvarez, I. M. Marrucho, M. Aznar, J. A. P. Coutinho, J. Supercrit. Fluids 2010, 52, 258.
[4] L. M. Galan Sanchez, G. W. Meindersma, A. B. de Haan, Chem. Eng. Res. Des. 2007, 85, 31.
[5] B. E. Gurkan, J. C. de la Fuente, E. M. Mindrup, L. E. Ficke, B. F. Goodrich, E. A. Price, W. F. Schneider, J. F. Brennecke, J. Am. Chem. Soc. 2010, 132, 2116.
[6] C. Wang, X. Luo, H. Luo, D. Jiang, H. Li, S. Dai, Angew. Chem. Int. Ed. 2011, 50, 4918.
[7] N. M. Simon, M. Zanatta, F. P. dos Santos, M. C. Corvo, E. J. Cabrita, J. Dupont, ChemSusChem 2017, 10, 4927.
