The following systems spin from SILP technology:
- IL, which is not participating in the reaction directly, can be applied on the catalytically active solid (solid catalyst with ionic liquid layer, SCILL, Scheme 1, a). In this case the role of IL is to modify the surface, making it more homogenous, but also to enhance selectivity for certain transformations and to serve the protection of catalytic poisons. The variation of this technique is when solid catalytically active species is supported on inert solid support and then covered in IL (Scheme 1, b).
- IL phase, which is catalytically active, can be supported on inert solid material (supported ionic liquid catalysis, SILCA). The catalyst role can be played by IL itself, when TSIL is used. This case provides a very high concentration of the active sites in the ionic liquid phase (Scheme 1, c). Alternatively, variety of catalysts can be dissolved or dispersed in IL phase, including homogenous metal catalyst, organocatalysts, nanoparticles (supported ionic liquid nanoparticles, SILnP) and even enzymes (Scheme 1, d). IL in those cases provides a controlled environment for well-defined catalyst and therefore simplifies directed modification of the catalytic system for increase in selectivity and/or activity. Homogenous catalysts treated this was may benefit from increased stability, as often they will be surrounded by the tight solvent cage. This affords operation of those systems at higher temperatures.
- In the two above-mentioned cases, ILs are held on the surface by physisorption. While this is ideal for use as catalysts in gas-phase reactions, in the liquid phase care should be taken of the solvent and IL compatibility, because the solubility of IL in the reaction solvent would result in leaching and washing out of the IL phase. This problem can be circumvented by applying a monolayer of functionalised IL that is held on the surface by chemisorption (Scheme 1, e). The covalently attached IL is fixed on the surface and therefore no leaching is possible. At the same time, IL brings desired functionality to the surface.
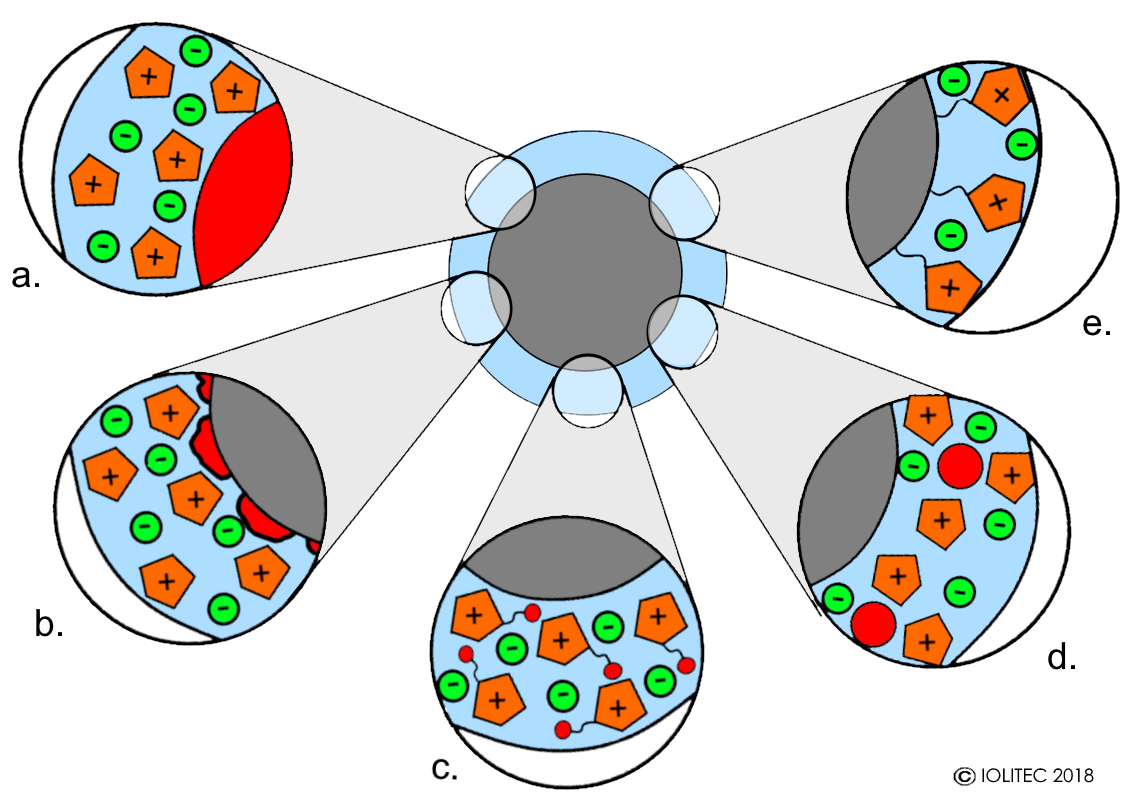
Scheme 1. Different variations of SILP: a. SCILL with catalytically active support; b. SCILL with heterogeneous catalyst on inert support and IL; c. SILP with functionalized ionic liquid bearing catalytically active groups; d. SILP with catalyst dissolved in ionic liquid phase; e. monolayer of IL covalently-bound to the support. Red color indicates catalytic centers.
To date, a variety of transformations was successfully performed in supported ILs, like C-C and C-N cross-coupling reactions, hydrogenations (using both homogenous and heterogeneous catalysts), enzymatic transformations and dynamic kinetic resolution, to name just a few.[1] SILP systems can also be employed in gas separation.
If you need assistance on choosing right ILs for your SILP application, please do not hesitate to contact us.
[1] R. Fehrmann, Supported Ionic Liquids: Fundamentals and Applications. Wiley-VCH, 2014.
