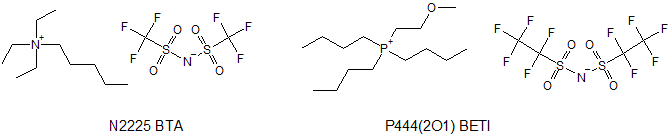
Recently, in the literature a quest for ideal ionic liquid for a salt bridge for measuring Gibbs energies of transfer was described.[1] And N2225 BTA appeared to be the winner – the ionic liquid with properties closest to mystical ideal. Before that, P444(2O1) BETI used to be the IL of choice for salt bridge in electrochemical measurements.[2]
But what defines the “ideal ionic liquid”? For the specific application of measuring chemical potential, the ideal IL must have
- High iconicity – most of it should exist as separate ions dissociated form each other.
- Identical transference numbers, or ionic mobilities, for cation and anion both neat and in solutions.
The former is usually the case for cations that are highly shielded by substituents and anions, that have ability to delocalize charge efficiently and/or sterically shielded – non-coordinating anions. The latter means that cation and anion of the ionic liquid should transfer exactly the same amount of charge, which is in most cases not the case not just for ionic liquids, but also for inorganic salts. Furthermore, the transference numbers normally depend on the solvent, as different solvation patterns are prevalent in different solvents.
N2225 BTA is very close to ideal. Its iconicity degree is 0.95,[3] molecular volumes of cation and anion are quite similar and self-diffusion coefficients are equal for cation and anion and the proportion does not change dramatically in water or acetonitrile.1b This makes N2225 BTA very useful for measuring Gibbs energies of transfer between solutions and avoiding uncertainties originating from liquid-junction potentials across the system.
Do you have another use of IL with ideal properties in mind? Need an ideal salt bridge? Contact us and we will be happy to help you obtain the ionic liquid you need.
Text by Dr. Svetlana Cadu
1 (a) V. Radtke, A. Ermantraut, D. Himmel, T. Koslowski, I. Leito, I. Krossing, Angew. Chem. Int. Ed. 2018, 57, 2344; Angew. Chem. 2018, 130, 2368; (b) A. Ermantraut, V. Radtke, N. Gebel, D. Himmel, T. Koslowski, I. Leito, I. Krossing, Angew. Chem. Int. Ed. 2018, 57, 2348; Angew. Chem. 2018, 130, 2372.
[2]T. Kakiuchi, Electrochem. Commun. 2014, 45, 37.
[3] A. Rupp, N. Roznyatovskaya, H. Scherer, W. Beichel, P. Klose, C. Sturm, A. Hoffmann, J. Tubke, T. Koslowski, I. Krossing, Chem. Eur. J. 2014, 20, 9794.
