The ionic liquid research community all over the world works hard to identify new applications of those unique materials. Today I would like to highlight the work form France and Czech Republic.[1] As a result of their research, porous liquid was prepared.
What is porous liquid?
As the name says, it is a material in the liquid state that has permanent porosity. In this case, when ionic liquid was mixed with metal-organic framework (MOF), a new structure was formed. It still bears some qualities of the parent liquid, however it also offers porosity, inherited from the MOF. The authors used easily available material to prepare their porous liquid: trihexyltetradecylphosphonium bis(trifluoromethylsulfonyl)imide (P666(14) BTA) and MOF Zn(2-methylimidazole)2 (ZIF-8). They choose just the right combination of starting materials: the ionic liquid molecules are big enough, that they cannot enter the cages formed by MOF. That results in a mixture with low density and stable pores.
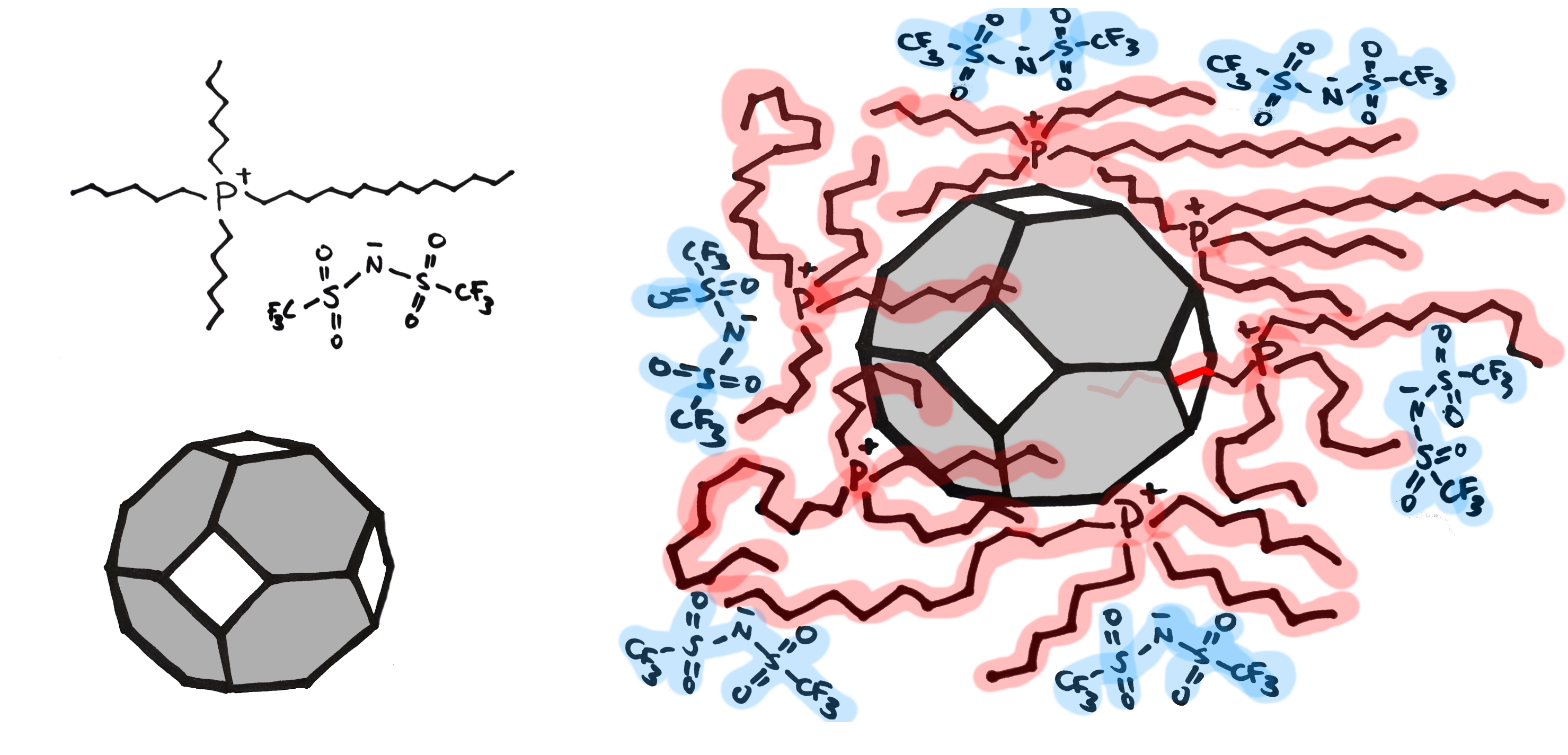
Scheme 1. Left: starting materials for the formation of porous liquid (P666(14) BTA and ZIF-8). Right: the structure of the formed porous liquid. The openings in the porous structure with inner pore size of 11.6 Å are only 3.4 Å in diameter (white rectangles in the scheme). This is too small for the molecule if IL to enter the structure, however occasional penetration of some of the alkyl chains can occur (red chain inside the void on the right).
What can porous liquids be useful for?
One can envision porous liquids as molecular reservoirs and their big advantage that, unlike pure MOFs, they can flow and therefore easier to handle in industrial setups. Authors of the article have shown that P666(14) BTA and ZIF-8 system is capable of absorbing large volumes of gases. For example, the CO2 absorption capacity increases by 63% in the porous liquid compared to the parent IL, whereas the absorption of methane and nitrogen nearly doubles! Other applications include gas separations and catalysis.
Background
Porous liquids were coined in 2007 by James et al.[2] They are divided in three groups: type I are pure liquids with permanent rigid cavities, type II comprises porous hosts dissolved in liquids and type III has porous solids dispersed in liquid matrix. In the past, ILs was successfully incorporated in both type I porous materials using surface modification of hollow silica shells[3] and synthesis of polymeric ligid IL structure,[4] and recently also type III, using ZIF-8 and butylpyridinium bis(trifluoromethylsulfonyl)imide BuPy BTA[5] or DBU-based IL.[6]
[1] M. Costa Gomes, L. Pison, C. Červinka, A. Padua, Angew. Chem. Int. Ed. 2018, 57, 11909.
[2] N. O'Reilly, N. Giri, S. L. James, Chem. Eur. J. 2007, 13, 3020.
[3] J. Zhang, S. H. Chai, Z. A. Qiao, S. M. Mahurin, J. Chen, Y. Fang, S. Wan, K. Nelson, P. Zhang, S. Dai, Angew. Chem. Int. Ed. 2015, 54, 932.
[4] J. S. Lee, H. Luo, G. A. Baker, S. Dai, Chem. Mater. 2009, 21, 4756.
[5] S. Liu, J. Liu, X. Hou, T. Xu, J. Tong, J. Zhang, B. Ye, B. Liu, Langmuir, 2018, 34, 3654.
[6] W. D. Shan, P. F. Fulvio, L. Y. Kong, J. A. Schott, C. L. D. Thanh, T. Tian, X. X. Hu, S. M. Mahurin, H. B. Xing, S. Dai, ACS Appl. Mater. Interfaces 2018, 10, 32.
