Gas scrubbing is a wide spread technology for removal of impurities from gasses. In wet scrubbing, a gas stream is passed through the scrubber and the impurities dissolve or react in the scrubber fluid and the level, to which impurities are removed depends on their solubility in scrubber fluid.
Ionic liquids have following advantages when used for gas scrubbing:
- ultralow volatility provides
- no losses due to evaporation
- no contamination of gas stream by scrubber fluid
- no release of VOCs to the atmosphere
- high solubilizing power
- broadens the spectrum of impurities to be removed
- allows more efficient removal
- high stability gives
- wider operational temperature range
- possibility to add additional reactants for more efficient scrubbing
- tunable properties allow to adjust the scrubbing fluid for specific use and increase selectivity
- additional process safety is achieved due to
- non-flammability of ionic liquids
- possible biocompatibility
- non-corrosiveness
Ionic liquids have been shown to remove SO2 and NOx from industrial flue gasses[1] and can be efficiently used as SILP materials for this purpose. SO2 has higher solubility in ionic liquids, than other flue gasses.[2] This suggests that ionic liquids could be used for removal of sulphur from biogas prior to use, or allow decreasing the sulphur content in boat exhaust, while using current high sulphur fuel. Another avenue for this application is stripping of catalytic poisons from natural gas prior to use in fuel cells. BMIM Cl can also efficiently remove Hg from the flue gasses, when used together with hydrogen peroxide.[3]
There is further interest in the use of supported ionic liquid membranes (SILM) for gas separations, as it is easier to incorporate membranes into already existing infrastructure. If one gas can be selectively absorbed within the membrane and further transported within it and released on the other site, the separation if this gas can be achieved. In such way, for examples, separation of ethylene from ethane can be performed with the BMPyrr BTA-based membrane doped with Ag BTA.[4]
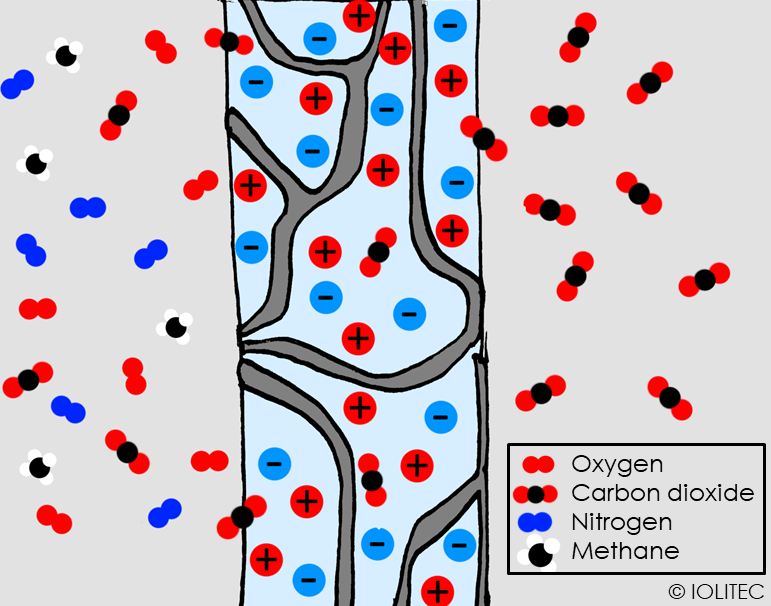
Scheme 1. Separation of CO2 from the gas mixture with SILM can be described in three steps: 1. Dissolution of CO2 into IL, that is trapped within polymeric matrix; 2. Diffusion of CO2 within the membrane; 3. Desorption on the other side of membrane.
If you are interested in using ionic liquids for gas scrubbing, please feel free to contact us for more information on specific products.
Text and illustrations: Dr. Svetlana Cadu, Iolitec 2019.
[1] P. K. Kaas-Larsen, P. Thomassen, L. Schill, S. Mossin, A. Riisager, R. Fehrmann, ECS Transactions, 2016, 75, 3-6.
[2] W. Wu, B. Han, H. Gao, Z. Liu, T. Jiang, J. Huang, Angew. Chem. Int. Ed., 2004, 43, 2415-2417.
[4] L. M. Galan Sanchez, G. W. Meindersma, A. B. Haan, Ind. Eng. Chem. Res. 2009, 48, 10650-10656.
