Designer solvent
It is widely accepted, that properties of ILs can be fine-tuned comparatively easily, by varying substituents on cations and anions. This allows constructing the “right” ionic liquid for certain applications. A striking example is the difference between BMPyrr BTA and BMPyrr OMs for the nucleophilic aromatic substitution, with former giving 0.4% conversion, whereas latter afforded 77% under identical conditions. The researchers used rational approach based on the studies of solvent-solute interaction to find the “right” IL for the reaction.[1] This shows the tremendous potential of ILs, if the similar approach applied to large-scale or challenging reactions.
Ionic liquids playing the role of catalyst and solvent
The classical Friedel-Crafts reactions use volatile acid as catalysts. It has been shown that the role of acid can be played by ionic liquid solvents, for example tetrachloroaluminate based ionic liquids have been used in Friedel-Crafts reaction between benzene and phosphorous trichloride. It allowed for easy separation of the product and possible recycling of the catalyst. [2]
Another example of the use if IL as the solvent that also promotes the reaction is synthesis of hydroxymethylfurfural (HMF) – a platform chemical that is nicknamed “sleeping giant”, because it can be transformed to a broad spectrum of potentially useful compounds. When ionic liquids are used as solvents for this dehydration reaction, no additional acid is required. Furthermore, in IL with addition of CrCl2, HMF can be made from glucose directly (Scheme 1).[3]
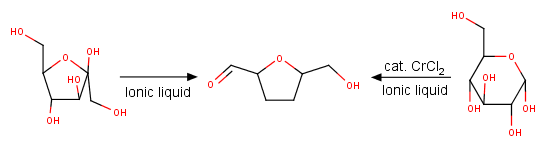
Scheme 1. Synthesis of HMF in ILs.
ILs as solvents for transition metal catalysed reactions
By now, probably most of the well-known name reactions have been conducted in ionic liquids. Those include Suzuki cross-coupling, Heck reaction, Stille coupling, olefin methatesis, hydroformylation, hydrogenation, epoxidation to name but a few. Imidazolium ILs in this context can react with transition metal derivatives to form active catalyst species (Scheme 2), but also to inhibit it.
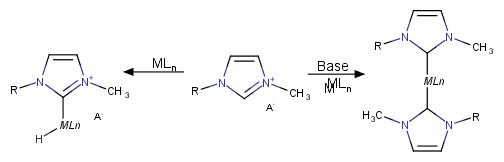
Scheme 2. Formation of metal complexes from ILs.
For example, in carbonylation of ethylene, as shown in Scheme 3, the use of ILs with Brønsted acid SO3H functionality stabilized the palladium catalyst, preventing it from precipitating as palladium black, but also resulted in biphasic reaction mixture, which facilitated the product separation and allowed the reuse of precious metal catalyst.[4]
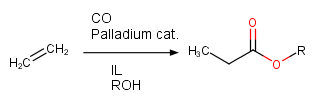
Scheme 3. Carbonylation of ethylene in IL.
Another example of a positive influence onto reaction outcome is oxidation of primary alcohols to aldehydes. Quite often, over-oxidation is a problem in those reactions, but it has been shown, that when CuCl catalyzed oxidation is performed in BMIM BF4, no over-oxidation is observed as long as the solvent is dry to moderately wet.[5]
Asymmetric reactions could also be successfully conducted in ILs, as illustrated by allylation of amines in ILs. Pyrrolidinium ILs gave yield and enantioselectivity that is superior, compared to other classes of ILs or molecular solvents and allowed for the reuse of the catalyst.[6]
Another elegant study used IL-water-hexane triphasic system to conduct Heck reaction: the Pd-catalyst (as tetrachloropalladate) remained in the IL phase, the product extracted into hexane phase, whereas salt by-product went to aqueous phase, which facilitated the separation greatly.[7]
ILs as solvents for organo-catalysed reactions
There is a plethora of proline derivatives that are often used as catalysts in asymmetric transformations. Often, those reactions have to be done at low temperatures, to ensure the highest possible level of enantioselectivity, but of course, it slows down the reaction. The use of IL as a solvent for the aldol reaction of aldehydes with ketones allowed to conduct the reaction at 0 ⁰C, increasing the yield without deterioration of ee. The role of IL is supposed to be in enhancement of nucleophilicity of enamine and stabilisation of the iminium intermediate.[8]
ILs as solvents for enzyme catalysis
Lipases are the most widely-used enzymes in organic chemistry, with Candida antarctica lipase B (CALB) being perhaps the most prominent example. It has been shown that when kinetic resolution can be performed in BMIM PF6, the enzyme can be recycled (Scheme 4).[9]
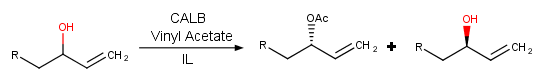
Scheme 4. Kinetic resolution in IL.
Interestingly, CALB-catalysed epoxidation of cyclohexene can be conducted using hydrogen peroxide and octanoic acid in IL, achieving peracid formation in situ, followed by epoxidation. Compared to the usual use of peracid, the reaction in IL is safer, as it circumvents the use of hazardous peracid and flammable solvents, achieving comparable result.[10]
ILs as solvents for whole cell biocatalysis
The step on the way to greener catalysis is application of whole cell as catalysts, as it does away with sometimes difficult and costly purification of the enzymes. One example of that is the hydrolysis of nitriles into amides, which is of industrial importance. This reaction proceeds very well in the IL-water biphasic system catalysed by Rhodococcus R312 cells. It is quite remarkable that ILs did not reduce cell viability and, in addition, the reduced cell aggregation at the interface made the product isolation easier.[11]
The advantageous values of the partition coefficients between ILs and water allowed minimizing the non-catalytic hydrolysis of epoxides and achieving higher enantioselectivities and yields in mung bean catalysed reaction. The result was much better that in aqueous system.[12]
ILs in some industrially important processes
The petrochemical industry conducts alkylation of isobutane with butene to get fuels with higher octane numbers. This reaction is normally catalysed by HF, a dangerous and toxic substance. However tetrachloroaluminate IL can be used instead of HF as a catalyst in this Chevron process.[13]
Monsanto and Cativa processes use carbonylation of methanol to form acetic acid on industrial scale, using Rh and Ir catalysts respectively. The separation of expensive catalysts from those main-stream chemicals can be difficult. It was proposed, that silica supported ionic liquid rhodium catalyst can be used in the Monsanto-like process. The catalyst immobilised in IL phase exhibited excellent activity and selectivity.[14]
[1] I. Newington, J. M. Perez-Arlandis, T. Welton, Org. Lett. 2007, 9, 5247.
[2] Z.-W. Wang, L.-S. Wang, Appl. Catal. A. 2004, 262, 101.
[3] C. Fayet, J. Gelas, Carbohydr. Res. 1983, 122, 59.
[4] E. J. Garcıa-Suarez, S. G. Khokarale, O. N. van Buu, R. Fehrmann, A. Riisager,
Green Chem. 2014, 1, 161.
[5] I. A. Ansari, R. Gree, Org. Lett. 2002, 4, 1507.
[6] I. Favier, A. B. Castillo, C. Godard, S. Castillón, C. Claver, M. Gómez, E. Teuma, Chem. Commun. 2011, 47, 7869.
[7] A. J. Carmichael, M. J. Earle, J. D. Holbrey, P. B. McCormac, K. R. Seddon, Org. Lett. 1999, 1, 997.
[8] H. M. Guo, L. F. Cun, L. Zh. Gong, A. Q. Mi, Y. Z. Jiang, Chem. Commun. 2005, 1450.
[9] T. Itoh, E. Akasaki, K. Kudo, S. Shirakami, Chem. Lett. 2001, 30, 262.
[10] R.M. Lau, F. van Rantwijk, K.R. Seddon, R.A. Sheldon, Org. Lett. 2000, 2, 4189.
[11] S.G. Cull, J.D. Holbrey, V. Vargas-Mora, K.R. Seddon, G.J. Lye, Biotechnol. Bioeng. 2000, 69, 227.
[12] C. Chiappe, E. Leandri, B. D. Hammock, C. Morisseau, Green Chem. 2007, 9, 162.
[13]A. Corma, A. Martinez, Catal. Rev. 1993, 35, 483.
[14] Q. Riisager, B. Jorgensen, P. Wassersheid, R. Fehrmann, Chem. Comm. 2006, 994.
