Ionic Liquids as Solvents for Polymers
The use of ionic liquids as reaction medium in organic synthesis and catalysis was suggested very early. The general interesting combination of properties and the fact that properties can be tailored by design, opened already the door to applications in the field of organic, but also biochemistry and inorganic chemistry. Why also should one not use them in polymer chemistry?
The start of ionic liquids in polymer research was anything else but a good one: In the mid 1990s Watanabe and coworkers described the insolubility of poly(MMA), poly(St), poly(AN) and poly (EO) in butylpyridinium bromide - AlCl3.1 In 2000 Forsyth et al. were able to dissolve poly(N,N-dimethyl acrylamide), poly(DMMA), poly(AN), poly(1-vinylpyrrolidinone), and poly(VPyr-coVAc) in Trimethylbutylamonium bis(trifluoromethylsulfonyl)imide (N1114 BTA).2 A couple of other solubility studies were made, but a first milestone maybe was the work from Winterton et al., who presented in a comprehensive study the behavior of 17 different polymers over 11 weeks in the ionic liquids 1-ethyl-3-methylimidazolium tetrafluoroborate (EMIM BF4), 1-butyl-3-methylimidazolium hexafluorophosphate (BMIM PF6) and 1-methyl-3-octylimidazolium bis(trifluoro-methylsulfonyl)amide (OMIM BTA). By far the most polymers were dissolved in OMIM BTA.3 The rough trend that was identified was that hydrophilic polymers were soluble in all three ionic liquids, while the more hydrophobic polymers were insoluble or only soluble in OMIM BTA. The reason for the enhanced solubility in OMIM BTA may be caused by the different nature of anion: In general the physical and chemical properties of ionic liquids are dominated more by the anion. As Winterton et al. mentioned, BF4 and PF6, which are both non-coordinating anions, showed a more or less similar behavior. The BTA-anion on the other hand is a weak coordinating anion, leading typically to a behavior which is more similar to hydrophobic molecular solvents.
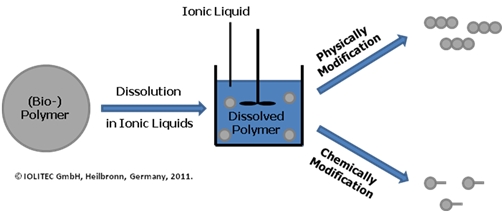
Figure 1. Dissolution of Polymers in ionic liquids.
In this context, another important field that should not be neglected is the dissolution of biopolymers: Robin Rogers, one of the important door openers in ionic liquids research, demonstrated in 2002 that the by far most important biopolymer cellulose could be dissolved in some ionic liquids better than in any other solvents.4 Once this biopolymer is dissolved it can be processed by chemical of physical methods to produce novel chemicals or materials. As a consequence, it is no wonder that the dissolution of cellulose is surely at the moment one of the most important fields of ionic liquids research, since novel perspectives in the field of biomass-to-liquid transformations are created.
If you are interested in this topic, please do not hesitate to contact us!
Text: Dr. Thomas J. S. Schubert, IOLITEC GmbH, 2012.
[II C5]
1. M. Watanabe, S. Yamada, K. Sanui and N. Ogata, Chem. Commun. 1993, 929.
2. M. Forsyth, S. Jiazeng and D. R. MacFarlane, Electrochim. Acta 2000, 45, 1249.
3. N. Winterton, J. Mater. Chem. 2006, 16, 4281.
4. R P. Swatloski, S. K. Spear, J. D. Holbrey, R. D. Rogers, J. Am. Chem. Soc. 2002, 124, 4974.
