Zinc-air batteries are widely used as power supply for hearing aids, but only as primary batteries. The general principle of Zn-air battery is illustrated in scheme 1. While those batteries potentially have a great benefit of low price and wide availability of anode material[1], in addition to producing stable voltage, attempts to commercialize secondary Zn-air batteries meet certain difficulties.
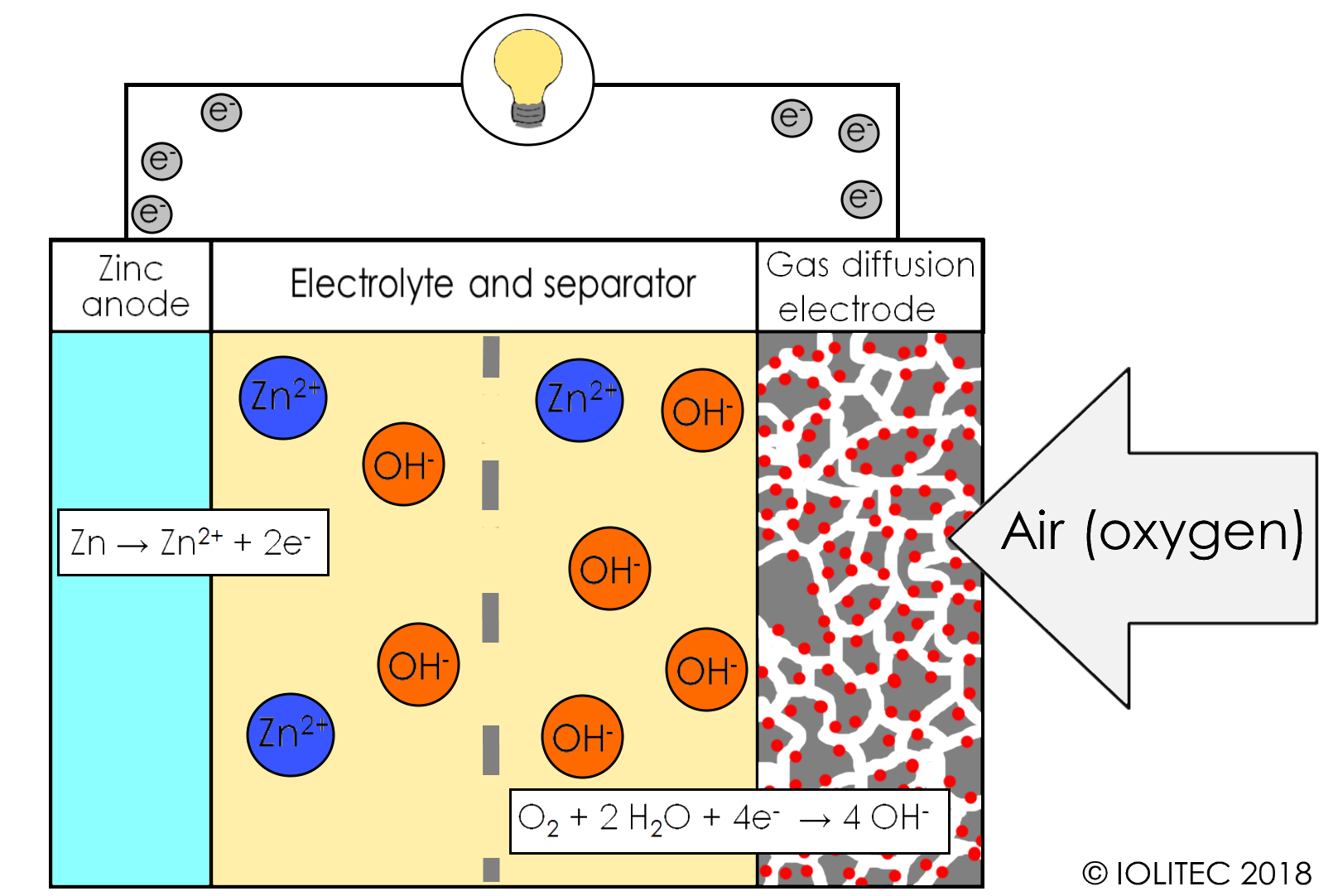
Scheme 1. General working principle of zinc-air battery.
Firstly, cathodes of metal-air batteries are very sensitive and require often expensive catalyst (red dots in scheme 1), they often fail due to either flooding or drying over, depending on atmospheric humidity. Secondly, current state-of-the-art electrolyte (aqueous KOH solution) does not show the stability required in a long run, as it is prone to lose water and react with CO2 from the air to form carbonate, which results in conductivity drop. Furthermore, during recharging process, dendrites are often formed, leading to the short circuit and posing safety risks. Formation and precipitation of zinc oxides and carbonates leads to a loss of active material and blockage of the gas diffusion electrode surface. Furthermore, superoxide ions formed during electrochemical reactions on cathode lead to the decomposition of electrolyte. The problems of Zn-air batteries are summarized in scheme 2.
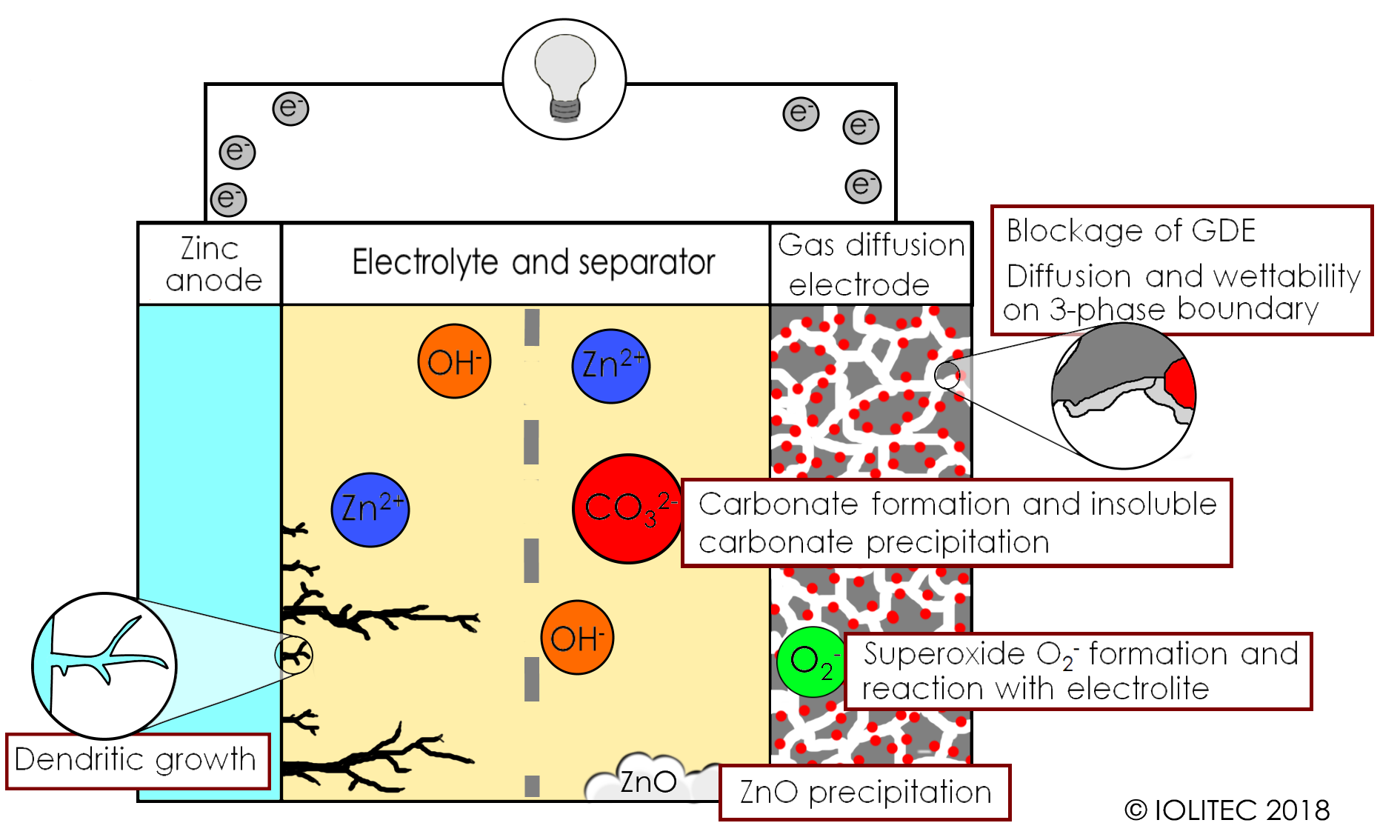
Scheme 2. Challenges on the way to rechargeable Zn-air batteries.
Compared to classical electrolytes, ionic liquids can be designed to overcome the above mentioned problems. For example, most of ionic liquids don’t react with CO2. The ultralow vapour pressure of ILs makes evaporation of water less likely, protecting cathode from drying. As for the case for lithium sulphur batteries, ionic liquids can suppress dendritic growth. Furthermore, some ionic liquids can further improve the performance of the batteries but providing better solubility of zinc derivatives and therefore minimizing loss of active species by precipitation. Some ILs also offer benefits of high O2 solubility, low toxicity, and compatibility with other materials of the battery. For example, some ionic liquid might be used for protection of electrodes from corrosion.
Within the framework of LuZi project[2], we explored ionic liquids as electrolytes for zinc-air batteries and found that the best performance could be achieved by using mixtures of ILs and water. However, this type of batteries requires further development. If you are interested in using ionic liquids for Zn-air batteries and want to discuss this topic, please do not hesitate to contact us.
Text and illustration: Dr. Svetlana Cadu, 2018
[1] https://www.metalary.com/, retrieved on 12.06.2018
[2] Project “LuZi”, FKZ 03SF0499G
(„LUZI“– Zink/Luft-Batterien mit neuartigen Materialien für die Speicherung regenerativer Energien und die Netzstabilisierung.)
Project period: 01.07.2015 – 30.06.2018
